2. METHODOLOGY
2.1 Scope and purpose
The need for these guidelines
The MEC is a device with a definite role in the treatment of male UI, but is probably underused because of a lack of education. These guidelines are intended to fill the gap of (evidence-based) information and encourage healthcare professionals to consider this option more often.
Overall objective
These guidelines provide guidance to healthcare professionals, patients and their families for the correct assessment and standard use of MECs in men with UI. The aim is to expand knowledge regarding MEC products and provide practical help in using them.
The guidelines were developed to prevent unintended harm to patients and to enhance compliance with using MECs. We describe evidence-based or best practice for safe use of MECs based on the literature search and consensus decisions in the Working Group. The Group decided to include topics such as indications, contraindications and alternatives, nursing principles, and interventions in male external catheterisation, as well as patient education. They also included what was found on aspects that have influence on quality of life (QoL).
Expected benefits
The scope of these guidelines was established at the start of the writing process. Six PICO questions were posed to guide the literature review process (Chapter 14).
These guidelines, in which we include clear illustrations, detailed application procedures and extensive references, will help them to identify potential problem areas in the assessment, application and removal of MECs.
More specifically, these guidelines aim to support healthcare professionals in the prevention of complications of male external catheters, such as UTIs, irritative and allergic symptoms, compressive symptoms and pressure sores, skin lesions and leakage, and contribute to improving the QoL of MEC users.
Although these guidelines aim to be comprehensive, effective practice in assessing MECs and supporting patients who are going to use these devices can only be achieved if the nurse or practitioner has a clear and thorough knowledge of the urinary tract anatomy, the necessary understanding of basic nursing principles, and has been assessed in practice as competent in this procedure.
These guidelines are expected to have an impact on men with UI who could benefit from (partially) using MECs.
Limitations
The EAUN Guidelines Working Group has prepared these guidelines to help nurses assess evidence-based management and incorporate the recommendations into their clinical practice. These guidelines are not meant to be prescriptive, nor will adherence to them guarantee a successful outcome in all cases. Ultimately, decisions regarding care must be made on a case-by-case basis by healthcare professionals after consultation with their patients and colleagues, and using their clinical judgement, evidence-based knowledge, and expertise.
Composition of the team
The Working Group of these updated guidelines consists of nurse specialists Veronika Geng, Susanne Vahr and Hanny Cobussen-Boekhorst with support from Hanneke Lurvink from the EAUN Central Office and urologist Ian Pearce for the Indications chapter.
2.2 Literature search
The information offered in these guidelines was obtained through a systematic literature search and a review of current procedures undertaken in various member countries of the EAUN.
The initial search was done in December 2014 by Veronika Geng, Nurse Specialist, Germany.
Databases
- Pubmed
- Cinahl
- Cochrane
Search terms
- Male external catheters
- Condom catheters
- Urinary sheaths
- External urinary catheter
In July 2015 an additional search was performed by Susanne Vahr, Nurse Specialist, Denmark.
Databases
- Embase
- Cinahl
- Cochrane
Search terms
- Male external catheters
- Condom catheters
- Urinary sheaths
- External urinary catheter
- Complications
As a result of the lack of medical subject headings, (MeSH) the searches were performed with free text for male external catheter and condom catheter as well as urinary sheaths.
The search results were not limited to randomised controlled trials, controlled clinical trials, meta-analyses or systematic reviews. Additional searches were not limited to any level of evidence (LE). For the practical aspects of MEC application (see Appendices), brochures from manufacturers were used.
2.3 Limitations of the search
The search and data extraction were based on PICO questions formulated by the Working Group (Chapter 14).
Limitations of December 2014 search:
- English language
- Adults
- Human studies
- Age ≥ 19 years
- 2004-2014
Exclusion criteria during abstract selection:
- Non-English-language studies
- Conference proceedings
- Paediatric studies
- Use of MECs for diagnostic reasons
It was a policy decision to restrict the search in the way described. After screening the records retrieved from the search of December 2014 (limited to 2004-2014), it was decided to do an additional search without limitation of year. However, it was decided not to use articles from before 2000 for the text on complications because those studies might have been performed with catheters made from material that is no longer used. After review, papers used in the original guidelines (2008) were included where text remained unchanged.
In the process of working with the articles, new references were found and added to the reference list, if they were relevant to the topic and cited in the text.
2.4 Search results
The searches resulted in:
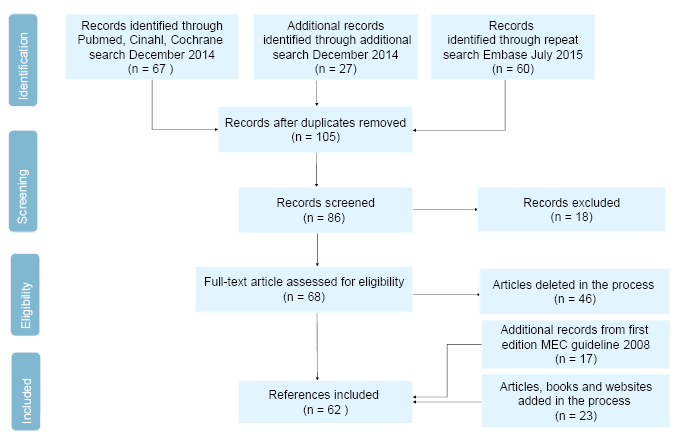
Flowchart 1. Literature search “Catheterisation - male external catheters in adults”
2.5 Disclosures
Members of the EAUN Guidelines Working Group have provided disclosure statements of all relationships that might be a potential conflict of interest. This information is stored in the EAU database. The EAUN is a non-profit organisation and funding is limited to administrative assistance and travel and meeting expenses. No honoraria or other reimbursements are provided. This guidelines document was developed with the financial support of Coloplast, Hollister Incorporated and Manfred Sauer GmbH.
2.6 Limitations of document
The EAUN acknowledges and accepts the limitations of this document. It should be emphasised that the current guidelines provide information about treatment of individual patients according to a standardised approach. The information should be considered as providing recommendations without legal implications. The intended readership is practising nurses and other healthcare professionals. Cost-effectiveness considerations are best addressed locally and therefore fall outside the remit of these guidelines.
2.7 Review process
A blinded review was carried out by specialist nurses, urologists in various countries and a patient representatives. The Working Group revised the document based on the comments received and included relevant references received (also from after the search period). A final version was approved by the EAUN Board and the EAU Executive responsible for EAUN activities.
2.8 Rating system
The recommendations provided in this document are based on a rating system modified from that produced by the Oxford Centre for Evidence-based Medicine (OCEBM) in 2011. [4] External data extractors used the EAU data-extraction system for critical assessment of the papers identified.
Whenever possible, the Working Group graded treatment recommendations using a three-grade system (grade of recommendation; GR A-C) and inserted levels of evidence to help readers assess the validity of the statements made. The aim of this practice is to ensure a clear transparency between the underlying evidence and the recommendations given. This system is further described in Tables 1 and 2. Much of the evidence is weak, therefore, the Working Group decided to upgrade some of the recommendations. Upgraded recommendations are marked ‘A*’ meaning that the panel has agreed to recommend this even though the LE is 4.
Some of the literature was not easy to grade. However, if the Working Group thought that the information would be useful in practice, it was ranked as LE 4. Low-level evidence indicated that no higher level of evidence was found in the literature when writing the guidelines, but it cannot be regarded as an indication of the importance of the topic or recommendation for daily practice.
The Working Group aims to develop guidelines for evidence-based nursing, as defined by Behrens (2004): “Integration of the latest, highest level scientific research into the daily nursing practice, with regard to theoretical knowledge, nursing experience, the ideas of the patient and available resources”. [5] The recommendations in these guidelines are based on synthesis of evidence from the articles. The Working Group based the text on the evidence of the articles whenever possible, but if evidence was missing, it was based on best practice and consensus.
Four components that influence nursing decisions can be distinguished: personal clinical experience of the nurse; existing resources; patient wishes and ideas; and results of nursing science. [5] This statement implies that, although literature is important, the experience of nurses and patients is also necessary for decision making. Consequently, it is not only the written guidelines that are relevant for nursing practice.
Table 1. Level of evidence (LE)
| 1a | Evidence obtained from meta-analysis of randomised trials |
| 1b | Evidence obtained from at least one randomised trial |
| 2a | Evidence obtained from one well-designed controlled study without randomisation |
| 2b | Evidence obtained from at least one other type of well-designed quasi-experimental study |
| 3 | Evidence obtained from well-designed non-experimental studies, such as comparative studies, correlations studies and case control studies |
| 4 | Evidence obtained from expert committee reports or opinions or clinical experience of respected authorities and case reports |
Adapted from Oxford Centre for Evidence-based Medicine (OCBM) [4]
Table 2. Grade of recommendation (GR)
| Grade | Type of evidence - nature or recommendation |
| A | Based on clinical studies of good quality and consistency addressing the specific recommendations and including at least one randomised trial |
| B | Base on well-conducted clinical studies, but without randomised clinical trials |
| C | Made despite the absence of directly applicable clinical studies of good quality |
Adapted from Oxford Centre for Evidence-based Medicine (OCBM) [4]
