2. Methodology
2.1 Guideline working group
The guidelines working group consisted of a multi-professional group of specialist nurses, and a medical colleague. Information about the authors can be found on page 56.
2.2 Literature search
The scientific basis of the information offered in these guidelines was obtained through a systematic literature search. All group members participated in the critical assessment of the scientific papers identified.
A search of the medical literature was conducted by Yuhong (Cathy) Yuan, Research Associate at McMaster University, Hamilton, Ontario, Canada. The initial search was conducted in July 2016 and repeated in November 2017 in the following databases:
- Cochrane Central Register of Controlled Trials
- Cochrane Database of Systematic Reviews
- Embase
- Epub Ahead of Print
- Ovid Medline(R) Daily and Ovid Medline(R)
Both medical subject headings (MeSH) and free-text terms, as well as variations of root words, were searched. The search was based on the keywords listed below.
2.3 Limitations of the search
PICO questions describe the four elements of a good clinical question, namely patient/problem, intervention, comparison, and outcome. For this guideline four PICO questions were defined (see 2.4). and the studies resulting from the search that seemed relevant for each question were evaluated.
The search results were not limited to randomised controlled trials (RCTs), controlled trials, meta-analyses or systematic reviews. In all databases, output was limited to human studies, adults aged >19 years, and English-language publications. The initial search was limited to 1 January 2010 until 23 July 2016, and the repeat search to 23 July 2016 until 28 November 2017.
Conference abstracts, editorial letters and case reports were excluded during the search.
2.4 PICO questions
PICO 1
In TRUS-guided prostate biopsy should the number of biopsy cores to find initial prostate cancer, independent of prostate volume, be between 10 and 12 cores, or more or less?
PICO 2
Is there any evidence that concomitant use of oral anti-coagulants influences the rate of bleeding complications in patients who undergo TRUS-guided prostate biopsy?
Is there any evidence that discontinuation of novel oral anti-coagulants influences the rate of bleeding complication in patients who undergo TRUS-guided prostate biopsy?
PICO 3
Is there any evidence that giving patient information before undergoing TRUS-guided prostate biopsy improves quality of life and patient experience, and reduces physical or psychological side effects?
Is there any evidence that giving information to patients after undergoing TRUS-guided prostate biopsy improves quality of life and patient experience, and reduces physical or psychological side effects?
PICO 4
Is there any evidence of an effect on quality, safety, outcome, follow-up or patient satisfaction of a nurse (specialist, practitioner, oncology) or physician assistant performing TRUS-guided prostate biopsy compared to a urologist or trainee performing TRUS-guided prostate biopsy.
2.5 Search keywords
The reference search included the following keywords:
- TRUS
- ultrasound
- prostate
- biopsy
- not: cancer related terms
2.6 Search results
Flowchart 1. Literature search “TRUS prostate biopsy”
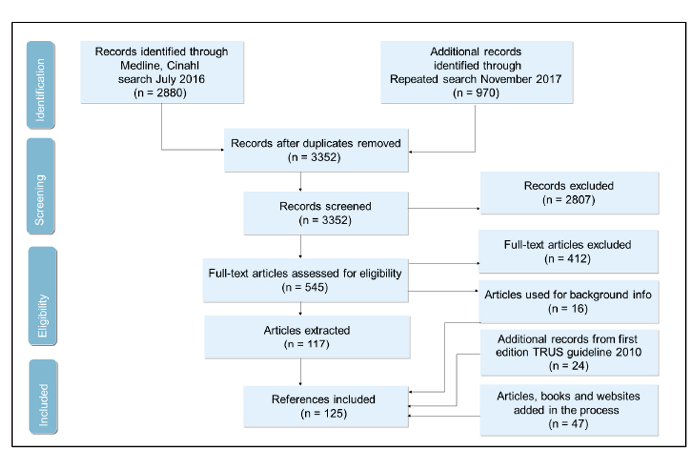
Numbers of records identified in search and update search
PICO 1: prostate biopsy and ultrasound, remove cancer terms; n = 3261 (2393 + 868)
PICO 2 = PICO 1 AND anti-coagulants; or anti-coagulants + urologist; n = 342 (280+ 62) (of them, 36 already captured by PICO 1).
PICO 3 = PICO 1 AND education = 143 (107+36) (all included in PICO 1);
PICO 4 = PICO 1 AND nurse: n = 16 (12+4) (all included in PICO 2);
CINAHL revised: n = 109 (88+21) (same search as for PICO 1 or anti-coagulants + urologist in Ovid)
It was a policy decision to restrict the search in the way described. Adding more keywords would have resulted in missing studies. In the process of working on the guidelines, some new references were found and added to the reference list, if they were relevant to the topic and cited in the text.
Screening and data extraction of the papers
Two panel members screened each abstract and two screened each full-text paper in Covidence. The most relevant studies were extracted. For PICO 2, 3 and 4 there were few relevant studies. For PICO 1 31 papers were extracted, and for Complications 67 papers were extracted. All extractions were performed in an Excel sheet. Systematic reviews were not extracted but reviewed separately during the writing process.
2.7 Exclusion criteria when selecting the abstracts
- transperineal prostate biopsy
- MRI
- saturation
- abstract
- studies written in a language other than English
- duplicates
- guidelines
2.8 Disclosures
All members of the EAUN guidelines working group have provided disclosure statements of all relationships that might be a potential source of conflict of interest. The information has been stored in the EAU(N) database.
The EAUN is a not-for-profit organisation and with the exception of administrative assistance, travel and meeting expenses, no honoraria or other reimbursements have been provided.
There was no external financial funding.
2.9 Limitations of document
The EAUN acknowledges and accepts the limitations of this document. Guidelines provide a standardised approach to patient care and management and practitioners must tailor care towards individual patients. The aim of guidelines is to help health care professionals to make informed decisions about their patients. Adherence to guidelines does not guarantee a successful outcome. Ultimately, health care professionals must make their own decisions about care on a case-by-case basis, using their clinical judgement, knowledge and expertise, and after consultation with their patients. Therefore these guidelines provide recommendations without legal implications.
Cost-effectiveness considerations and non-clinical questions are best addressed locally and therefore fall outside the remit of these guidelines. Other stakeholders, including patient representatives, have not been involved in producing this document.
When high-quality publications were lacking, the recommendations were based on expert reports or expert consensus. This is clearly indicated in the document.
2.10 Review process
Prior to publication, blinded review was carried out by 11 reviewers, including nurse specialists, two patients, an oncologist, an oncological pathologist and a urologist. After discussion of all comments received, appropriate revisions were made by the Working Group and the document was approved by the EAUN Board and the EAU Executive Board member responsible for EAUN activities.
2.11 Rating system
The recommendations provided in this document are based on a rating system modified from that produced by the Oxford Centre for Evidence-based Medicine (OCBM) in 2011. [10].
Whenever possible, the Working Group graded treatment recommendations using a three-grade system (grade of recommendation; GR A–C) and inserted levels of evidence (LEs) to help readers assess the validity of the statements made. The aim of this practice is to ensure a clear transparency between the underlying evidence and the recommendations given. This system is further described in Tables 1 and 2.
Some of the literature was not easy to grade. However, if the EAUN Working Group thought that the information would be useful in practice, it was ranked as LE 4 and GR C. Low-level evidence indicated that no higher level evidence was found in the literature when writing the guidelines, but cannot be regarded as an indication of the importance of the topic or recommendation for daily practice.
The literature used in these guidelines included one qualitative study, but no recommendation was made based on this study.
The recommendations in these guidelines are based on a synthesis of evidence from the articles.
The Working Group aims to develop guidelines for evidence-based nursing, as defined by Behrens (2004) [11]: “Integration of the latest, highest level scientific research into the daily nursing practice, with regard to theoretical knowledge, nursing experience, the ideas of the patient and available resources”. The Working Group based the text on evidence whenever possible, but if evidence were missing, it was based on best practice.
Four components that influence nursing decisions can be distinguished: personal clinical experience of the nurse, existing resources, patient wishes and ideas, and results of nursing science. [11] This statement implies that, although literature is important, the experience of nurses and patients is also necessary for decision making. Consequently, it is not only the written guidelines that are relevant for nursing practice.
Table 1. Level of evidence (LE)
| Level | Type of evidence |
| 1a | Evidence obtained from meta-analysis of randomised trials |
| 1b | Evidence obtained from at least one randomised trial |
| 2a | Evidence obtained from one well-designed controlled study without randomisation |
| 2b | Evidence obtained from at least one other type of well-designed quasi-experimental study |
| 3 | Evidence obtained from well-designed non-experimental studies, such as comparative studies, correlation studies and case reports |
| 4 | Evidence obtained from expert committee reports or opinions or clinical experience of respected authorities |
Adapted from the Oxford Centre for Evidence-Based Medicine (OCBM) [10]
Table 2. Grade of recommendation (GR)
| Grade | Nature of recommendations |
| A | Based on clinical studies of good quality and consistency addressing the specific recommendations and including at least one randomised trial |
| B | Based on well-conducted clinical studies, but without randomised clinical trials |
| C | Made despite the absence of directly applicable clinical studies of good quality |
Adapted from the Oxford Centre for Evidence-Based Medicine (OCBM) [10]
